Bremelanotide
Peptide Therapy in Pasadena
What Is Bremelanotide?
Bremelanotide, known commercially as Vyleesi®, is a synthetic peptide that activates melanocortin receptors involved in regulating sexual desire and neuroendocrine signaling.
FDA Approval: Vyleesi® is FDA-approved for premenopausal women with acquired, generalized hypoactive sexual desire disorder (HSDD).
Some clinicians also use Bremelanotide off-label in men for concerns such as low libido or erectile function, although these uses are not FDA-approved and research is ongoing.
At Alpha Hormones® in Pasadena, patients can receive educational consultations with licensed medical providers to better understand the science, safety, and responsible use of Bremelanotide.
Why Bremelanotide Therapy
Is Gaining Attention?
Bremelanotide has become an area of growing interest in sexual wellness and neuroendocrine medicine. Through its effects on the melanocortin system, it helps modulate signals that influence libido and mood.
Clinical and research areas include:
- Sexual desire and arousal: FDA-approved use in premenopausal women with HSDD.
- Mood and neuroendocrine balance: Ongoing studies evaluating effects on dopamine and stress pathways.
- Appetite regulation and metabolism: Early research exploring potential secondary effects on energy balance.
While FDA approval currently applies only to women with HSDD, emerging clinical discussions explore how men may also benefit from modulation of melanocortin pathways under professional supervision. Such uses are considered off-label and always require an individualized medical assessment.
Bremelanotide Therapy at Alpha Hormones® Pasadena
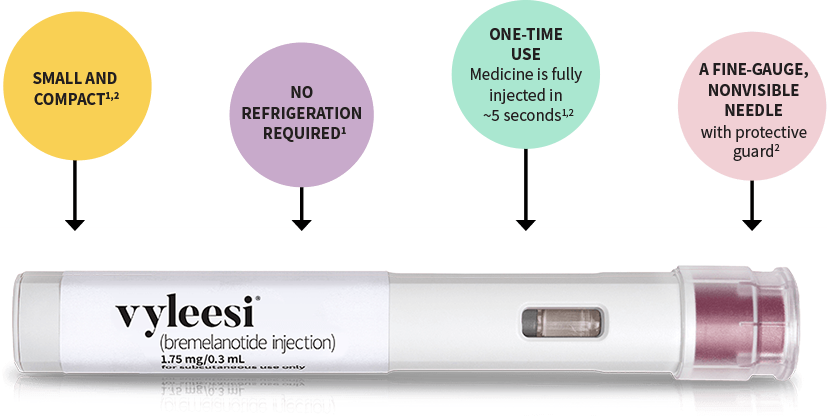
At Alpha Hormones®, we provide personalized consultations to determine whether peptide therapy—including Bremelanotide—aligns with your sexual wellness goals. Our licensed providers review FDA-approved indications, discuss off-label considerations when appropriate, and prioritize safe, medically guided care.
Available delivery methods include:
- Subcutaneous injection – self-administered before anticipated sexual activity.
- Intranasal spray – discussed as an alternative route for systemic absorption
- Oral troche – compounded forms that may also be combined with other sexual wellness medications, such as tadalafil or oxytocin, when clinically appropriate
What to expect: Some individuals report transient effects within hours, while others experience more gradual changes. Commonly reported temporary side effects include mild nausea, flushing, or headache. All potential reactions are reviewed prior to administration. Our Bremelanotide formulations are sourced only from GMP-compliant, licensed pharmacies, ensuring consistency and safety.


Who May Consider Bremelanotide Therapy?
Individuals who explore Bremelanotide therapy at Alpha Hormones® are often those who:
- Experience low sexual desire and want to understand options such as FDA-approved Vyleesi®
- Are open to discussing evidence-based, peptide-driven approaches to intimacy and confidence
- Seek professional, discreet, and medically guided support
Every patient begins with a private consultation to review health history, hormones, and goals. Our providers create an individualized plan focused on safety, education, and empowerment.
Frequently Asked Questions

Yes — Bremelanotide (Vyleesi®) is FDA-approved for the treatment of hypoactive sexual desire disorder (HSDD) in premenopausal women. All other uses, including in men, are considered off-label and must be discussed with a licensed medical provider.
It is a melanocortin receptor agonist, meaning it influences neural pathways related to sexual desire and arousal, rather than acting on hormones directly.
Typically as a subcutaneous injection before anticipated intimacy. Some compounding pharmacies may also prepare intranasal or oral formulations for research or wellness contexts.
Some individuals report noticeable changes within hours, but experiences vary. Bremelanotide should be used only under professional guidance.
The most common side effects include temporary nausea, flushing, and mild headache. Most are short-lived and dose-related.
While not FDA-approved for men, Bremelanotide’s mechanism of action is not sex-specific. Some clinicians discuss off-label use for men in select cases, always with informed consent and medical oversight.
In some cases, yes — especially in compounded troche form. Providers may combine Bremelanotide with medications such as tadalafil or oxytocin if clinically appropriate. This should only be done under licensed medical supervision.
Not necessarily. It may not be appropriate for individuals with uncontrolled hypertension, cardiovascular disease, or certain medication interactions. A medical consultation is required.
All formulations are obtained from licensed, GMP-compliant U.S. pharmacies to ensure consistency, purity, and safety.
Testing is often recommended to rule out hormonal or metabolic causes of low libido and to ensure safe use of peptide-based therapies.
Coverage varies. FDA-approved Vyleesi® may be covered under certain plans for eligible women. Compounded or off-label formulations are typically self-pay.
Yes. In most cases, patients can use FSA or HSA funds for medically supervised peptide consultations and peptide prescriptions, as long as they are ordered by a licensed medical provider and documented as part of a treatment plan.
Schedule a Private Consultation
At Alpha Hormones® in Pasadena, we specialize in hormone and peptide-based wellness with an emphasis on individualized care, safety, and transparency.
Interested in Bremelanotide (Vyleesi®)?
Schedule a confidential consultation with one of our licensed medical providers to explore whether this therapy may be an appropriate option for your personal wellness goals.
Disclaimer
Bremelanotide (Vyleesi®) is FDA-approved only for premenopausal women diagnosed with hypoactive sexual desire disorder (HSDD). All other uses are considered off-label. The information provided is for educational purposes only and is not a substitute for professional medical advice. Individual responses vary, and no outcomes are guaranteed. All treatment decisions should be made in consultation with a licensed healthcare provider following a personalized evaluation.





