Stem Cell Therapy in Pasadena
What Are Stem Cells?
Understanding Their Role in Regenerative Medicine
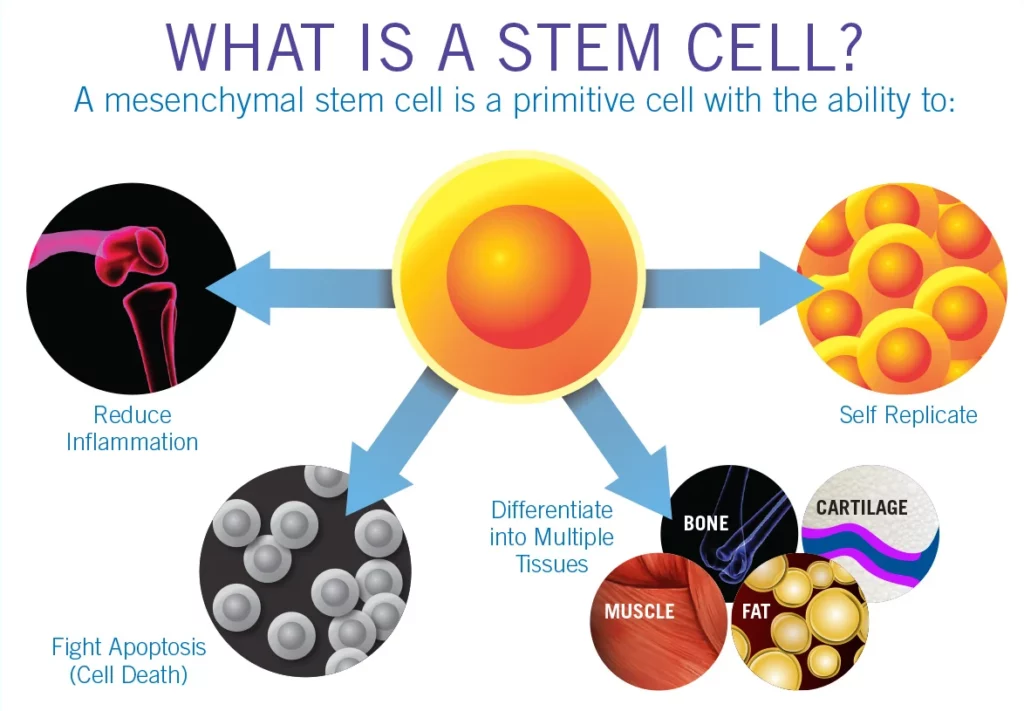
Stem cells are undifferentiated cells naturally found in the body. They are unique because of their ability to:
- Differentiate into specialized cell types (e.g., muscle, bone, or nerve).
- Release growth factors and signaling molecules that may influence cellular environments.
- Self-renew, making them potential building blocks in tissue support and repair.
Scientists are actively studying stem cells in regenerative medicine for their investigational potential. While research is ongoing, no stem cell therapy is FDA-approved to diagnose, treat, cure, or prevent disease beyond established uses in blood disorders.
At Alpha Hormones® in Pasadena, California, our role is to provide education, transparency, and personalized consultations so patients can better understand this evolving field.
Why Stem Cell Therapy Is Generating Interest in Regenerative Medicine?
Researchers worldwide are exploring how stem cells may play a role in supporting the body’s natural processes. Current investigational areas include:
- Cell differentiation → how stem cells may transform into specific types.
- Growth factor secretion → whether stem cells release molecules that influence repair environments.
- Tissue regeneration → exploring their role in natural recovery processes.
⚠️ Important Note: All of these applications remain investigational. Stem cell therapies are not FDA-approved for any medical use outside of hematopoietic (blood-forming) indications.
Educational Overview: Umbilical Cord–Derived Regenerative Components
Over the past several years, umbilical cord–derived biologic materials have attracted growing scientific interest within the field of regenerative medicine. These materials—sometimes referred to as perinatal tissue–derived components—are being studied for their signaling molecules and potential roles in cellular communication and tissue biology. Current research focuses on understanding their biologic characteristics rather than making claims of clinical benefit.
Commonly used biologic sources include:
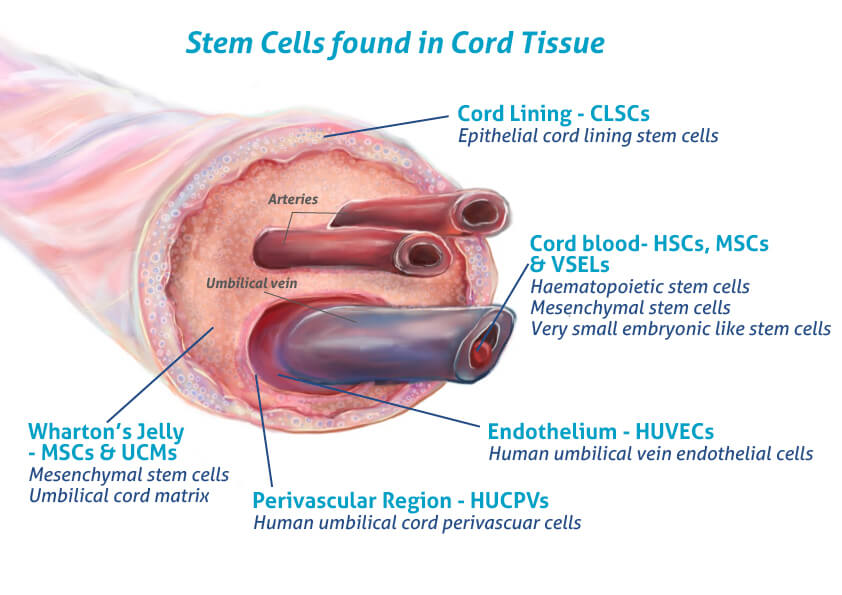
- Umbilical Cord: A structure that connects the mother and fetus during pregnancy. It contains structural cells and naturally occurring growth factors that are being examined for their biologic properties.
- Umbilical Cord Blood: A source of hematopoietic stem cells that are clinically used in FDA-regulated settings for certain blood and immune system–related conditions.
- Wharton’s Jelly: The gelatinous connective tissue within the umbilical cord. It contains mesenchymal stromal cells (MSCs), cytokines, and extracellular matrix proteins that are being studied in regenerative medicine research.
- Placental Membrane: A biologic fluid containing peptides, extracellular vesicles, and signaling molecules that are being studied for their roles in cellular communication.
- Amniotic Fluid: Rich in peptides and exosomes are being studies for roles in cellular communication and signaling.
Source classifications:
Biologic materials used in regenerative medicine research are commonly categorized based on their origin:
- Autogenic (Autologous): Derived from the patient’s own tissues, such as adipose tissue, bone marrow, or other autologous biologic preparations obtained and used within the same individual.
- Allogenic (Allograft): Derived from donated umbilical or placental tissue obtained from screened donors and processed according to established standards to remove cellular DNA and support safety and consistency.
Stem Cells: What’s FDA-Approved vs. Investigational?
FDA-approved uses:
- Hematopoietic stem cell transplants (bone marrow / cord blood) are FDA-approved for blood cancers such as leukemia, lymphoma, and multiple myeloma, and for certain inherited blood and immune disorders.
- These are hospital-based, highly regulated procedures.

Investigational uses:
- Stem cell–based approaches are being studied across multiple areas of medicine in investigational settings; evidence varies by condition and remains under active research.
- These uses are not FDA-approved and remain investigational.
National Regulatory Landscape for Stem Cell Therapy

In the U.S., stem cell therapies outside of hematopoietic transplants are considered investigational under FDA rules.
- As of 2025, regulatory requirements vary, and state-level frameworks do not replace federal FDA authority. While some states, including Florida, have enacted statutes or guidance addressing certain aspects of regenerative medicine practice, these measures do not supersede federal oversight, and investigational stem cell products outside of FDA-authorized pathways remain subject to federal regulation. Patients are encouraged to use caution with claims regarding the availability or legality of investigational stem cell products, particularly when such claims suggest approval, authorization, or routine clinical use.
Stem Cell Therapy Consultations at Alpha Hormones Pasadena
At Alpha Hormones® Pasadena, our regenerative medicine team provides comprehensive consultation services for individuals seeking information about biologic and regenerative medicine options. These consultations are designed to support informed decision-making through careful clinical evaluation and education.
During a consultation, our providers conduct a thorough review of relevant medical history, prior treatments, available imaging, and patient-specific considerations. The goal is to determine whether further discussion of regenerative medicine approaches may be appropriate within a clinical context.
As part of the consultation process, we may:
- Review health history, prior interventions, and available diagnostic studies
- Discuss general considerations related to autologous and allogeneic regenerative medicine approaches
- Outline potential timelines, monitoring considerations, and follow-up expectations, when applicable
- Evaluate overall hormonal and metabolic health as part of a broader clinical assessment
All services are provided on a case-by-case basis and do not imply treatment outcomes or FDA approval of any specific regenerative therapy. Information shared during consultations is intended to be educational and to support informed discussions between patients and licensed medical providers.
Process
Sourcing & quality
Monitoring
What patients report
Concierge Access to International Research Programs
In the United States, the use of stem cell–based approaches is generally limited to minimally manipulated cells, consistent with current federal regulatory frameworks. Regulatory standards and research environments may differ in other countries; however, such differences do not alter U.S. regulatory requirements or oversight.
At Alpha Hormones®, we provide educational consultations only for individuals seeking general information about stem cell research and international regulatory considerations. These consultations are intended to support informed discussion and understanding and do not involve the provision, coordination, referral, or endorsement of any specific stem cell therapy or treatment.
This may include:
- Providing general information about international research settings and regulatory environments
- Educating patients about investigational protocols in an informational context
- Discussing what is currently known, what continues to be studied, and areas where evidence remains limited or evolving
⚠️ These opportunities are outside the United States, regulated under their host countries, and remain investigational. They are not FDA-approved.
How Stem Cells and Exosomes Are Being Studied Together?
Researchers are exploring potential synergies between stem cells and exosomes:
- Stem cells → provide a living cellular foundation, with potential for differentiation and renewal.
- Exosomes → act as messengers, carrying proteins, RNA, and growth factors that influence cell communication.
In investigational and research settings, stem cells are sometimes studied as a foundational component, while exosomes are explored for their potential role in cellular signaling. Both approaches remain investigational, and scientific understanding continues to evolve.
At Alpha Hormones®, our providers offer educational consultations to help patients understand these concepts and to discuss general considerations related to options that may be available within their state or in international settings, depending on applicable regulations.

Patients who explore stem cell therapy often:
- Follow developments in regenerative and longevity medicine
- Want to learn about innovative but investigational options
- Value transparency and education from licensed providers
- Seek clear guidance on what is established versus still under research
Every patient begins with a dedicated one-on-one consultation to review their medical history, treatment goals, and the current state of stem cell research so they can make an informed decision.
Who Might Consider a Stem Cell Consultation?
Ethical & Safety Statement

Any references to stem cell products on this website are provided for general educational purposes and reflect common industry practices related to ethical sourcing and quality standards. Such products are typically described as being derived from full-term, healthy births with informed donor consent and processed in FDA-registered facilities with appropriate screening for sterility and quality. No embryonic or fetal tissue is involved.
Alpha Hormones® does not sell or commercially distribute stem cell products, and no content on this website represents stem cell therapies as FDA-approved products.
Stem Cell Therapy FAQs | Research, Safety, and Patient Education

In the United States, FDA approval for stem cell products is currently limited to specific blood-forming (hematopoietic) stem cell uses. Other stem cell-based approaches discussed in regenerative medicine research remain investigational.
In research and investigational settings, stem cells are studied for their role in cellular signaling, tissue development, immune response, and regenerative processes. Scientific understanding continues to evolve, and evidence varies by area of study.
In most cases, insurance does not cover stem cell therapy. Outside of specific FDA-approved uses (such as certain bone marrow or cord blood transplants), stem cell–related therapies are considered investigational and are typically not covered by private insurance or Medicare. Coverage varies by plan, and patients are encouraged to check directly with their insurance provider for details.
Exosomes are not cells — they are tiny vesicles secreted by cells that carry proteins, lipids, and genetic material to help with cell-to-cell communication. Unlike stem cells, exosomes cannot replicate or change DNA.
Some research is exploring combined approaches. Exosomes may amplify signaling, while stem cells may provide a cellular foundation. Both remain investigational.
No. Individual responses vary, and outcomes cannot be predicted or guaranteed. Many stem cell-based applications discussed in research settings have not been established as proven medical treatments.
Scientific literature commonly discusses autologous tissues and perinatal tissue-derived materials, such as umbilical cord or placental sources. These references are provided for educational purposes only and do not imply product availability.
When referenced in stem cell or perinatal tissue sourcing, cGMP (Current Good Manufacturing Practices) indicates that a manufacturer follows FDA-established standards for processing, handling, testing, and documentation. These standards are intended to support consistency, traceability, and quality control during manufacturing and storage.
cGMP compliance does not mean that stem cell or perinatal tissue products are FDA-approved or proven safe or effective for treating any medical condition. It relates only to manufacturing processes, not to clinical outcomes or regulatory approval for therapeutic use.
Yes. Stem cell research and clinical use are subject to federal regulatory oversight in the United States. Regulatory requirements may differ internationally; however, U.S. FDA authority remains the governing framework domestically.
No. While many of our patients are based in Pasadena, Los Angeles, and surrounding areas, we also see individuals who travel from outside the region. Telehealth consultations may be available depending on state regulations.
Schedule Your Stem Cell Consultation in Pasadena
At Alpha Hormones®, our mission is to guide patients through regenerative and wellness options with safety, transparency, and personalized care.
Interested in exploring stem cell therapy?
Book your consultation today with one of our licensed providers in Pasadena.
Disclaimer
Stem cell and exosome therapies are investigational and not FDA-approved to diagnose, treat, cure, or prevent any disease (except for certain blood cancers and immune disorders treated with hematopoietic transplants). Information is for educational purposes only and not medical advice. No outcomes are guaranteed, and individual results vary. Overseas treatments may involve manipulated stem cells from cGMP-certified facilities regulated under foreign laws, not the U.S. FDA.





